Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke
Michael L. Levy, MD, PhD; John R. Crawford, MD; Nabil Dib, MD; Lev Verkh, PhD; Nikolai Tankovich, MD, PhD; Steven C. Cramer, MD
Background and Purpose—Stroke is a leading cause of long-term disability. Limited treatment options exist for patients with chronic stroke and substantial functional de?cits. The current study examined safety and preliminary ef?cacy estimates of intravenous allogeneic mesenchymal stem cells in this population.
Methods—Entry criteria included ischemic stroke >6 months prior and substantial impairment (National Institutes of Health Stroke Scale score =6) and disability. Enrollees received a single intravenous dose of allogeneic ischemia-tolerant mesenchymal stem cells. Phase 1 used a dose-escalation design (3 tiers, n=5 each). Phase 2 was an expanded safety cohort. The primary end point was safety over 1-year. Secondary end points examined behavioral change.
Results—In phase 1 (n=15), each dose (0.5, 1.0, and 1.5 million cells/kg body weight) was found safe, so phase 2 subjects (n=21) received 1.5 million cells/kg. At baseline, subjects (n=36) averaged 4.2±4.6 years poststroke, age 61.1±10.8 years, National Institutes of Health Stroke Scale score 8 (6.5–10), and Barthel Index 65±29. Two were lost to follow-up, one was withdrawn and 2 died (unrelated to study treatment). Of 15 serious adverse events, none was possibly or probably related to study treatment. Two mild adverse events were possibly related to study treatment, a urinary tract infection and intravenous site irritation. Treatment was safe based on serial exams, electrocardiograms, laboratory tests, and computed tomography scans of chest/abdomen/pelvis. All behavioral end points showed signi?cant gains over the 12-months of follow-up. For example, Barthel Index scores increased by 6.8±11.4 points (mean±SD) at 6-months (P=0.002) and by 10.8±15.5 points at 12-months (P<0.001) post-infusion; the proportion of patients achieving excellent functional outcome (Barthel score =95) increased from 11.4% at baseline to 27.3% at 6-months and to 35.5% at 12-months.
Conclusions—Intravenous transfusion of allogeneic ischemia-tolerant mesenchymal stem cell in patients with chronic stroke and substantial functional de?cits was safe and suggested behavioral gains. These data support proceeding to a randomized, placebo-controlled study of this therapy in this population.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identi?er: NCT01297413. (Stroke. 2019;50:00-00. DOI: 10.1161/STROKEAHA.119.026318.)
Key Words: abdomen ? brain ischemia ? neuroprotection ? pelvis ? reperfusion
Stroke is perennially among the leading causes of human disability1 and the leading neurological cause of lost dis-ability-adjusted life years.2 The mean survival after stroke is 6 to 7 years, and indeed more than 85% of patients live past the ?rst year poststroke,3 many with years of enduring disability. Many restorative therapies are under study to improve outcomes after stroke.4 Restorative therapies aim to improve patient outcomes by promoting the neural processes under-lying behavioral recovery,5 and are distinguished from acute therapies, such as reperfusion or neuroprotection, that aim to reduce initial injury. As such, restorative therapies often have a time window measured in days-months, or in some cases6–9 in years.
Mesenchymal stem cells (MSC), also known as mes-enchymal stromal cells, are among the leading restorative therapy candidates. Substantial preclinical data support the safety and ef?cacy of MSC as a restorative therapy to improve outcomes after stroke. For example, a meta-analysis reported that 44 of 46 preclinical stroke studies found MSC to be supe-rior to placebo,10 with effect sizes >1.0.
Initial human studies of MSC (or MSC-like cells) after stroke focused on autologous cell therapies,11–13 whereby bone marrow is taken from each patient to produce his/her own MSC batch, and found MSC infusion to be safe. MSC are rela-tively immunoprivileged given their very low levels of human leukocyte antigen molecule expression,14 a fact that opens the door to administration of allogeneic MSC. Allogeneic MSC Table 1. Entry Criteria have been found to be safe without use of concomitant im-munosuppression,15 and can be manufactured in a manner that enables broad clinical application. Studies of allogeneic MSC (or MSC-like cells) poststroke have focused on early time points (administration 24–48 hours poststroke)16 or used an invasive procedure to implant cells intracerebrally.17 Each approach has its relative advantages and disadvantages, and an intravenous method of introducing MSC if comparably ef-?cacious might facilitate widespread implementation and also avoid adverse events attributable to invasive procedures.
The current study was a phase I/II dose-escalation trial that examined effects of a single intravenous infusion of al-logeneic ischemia-tolerant MSC. The target population was patients with chronic ischemic stroke and substantial func-tional de?cits, a group for whom treatment options remain limited. The primary outcome was safety, based on serial measures of behavior, computed tomography (CT) scans, and laboratory testing. Preliminary estimates of treatment ef?cacy were also examined.
Table 1. Entry Criteria
| Inclusion criteria |
| 1. Age =18 y |
| 2. Ischemic stroke =6 mo prior, radiologically confirmed at initial diagnosis and at study enrolment |
| 3. Severe disability resulting from the index stroke, operationally defined as subject confined to a wheelchair or required to have home nursing care or needing assistance with activities of daily living |
| 4. No substantial improvement in neurological or functional status for the 2 mo before study enrolment |
| 5. NIHSS score 6–20 |
| 6. Life expectancy >12 mo |
| 7. Patient receiving standard of care secondary stroke prevention before enrolment |
| 8. Patient or a surrogate able to provide informed consent |
| 9. Reasonable expectation that the patient will receive standard posttreatment care and attend all scheduled study visits |
| 10. Adequate systemic organ function, specifically: Serum aspartate aminotransferase =2.5× upper limit of normal Serum alanine aminotransferase =2.5× upper limit of normal Total serum bilirubin =1.5× upper limit of normal Prothrombin time and partial thromboplastin time =1.25× upper limit of normal in subjects who are not receiving anti-thrombotic therapy Serum albumin =3.0 g/dL Absolute neutrophil count =1500/µL Platelet count =150 000/µL Hemoglobin =9.0 g/dL Serum creatinine =1.5× upper limit of normal Serum amylase or lipase =1.0× upper limit of normal |
| Exclusion criteria |
| 1. History of uncontrolled seizure disorder |
| 2. History of cancer within the past 5 y, with the exception of localized basal or squamous cell carcinoma |
| 3. History of cerebral neoplasm |
| 4. Positive for hepatitis B, C, or HIV |
| 5. Myocardial infarction within 6 months of study entry |
| 6. Presence of any other clinically significant medical or psychiatric condition, or laboratory abnormality, for which study participation would pose a safety risk in the judgment of the Investigator or Sponsor |
| 7. Findings on baseline computed tomography suggestive of subarachnoid or intracerebral hemorrhage within past 12 mo. |
| 8. Participation in another investigational drug or device study in the 3 mo before treatment |
| 9. History within the past year of drug or alcohol abuse |
| 10. Pregnant or lactating, or expectation to become pregnant during the study |
| 11. Allergy to bovine or porcine products |
NIHSS indicates National Institutes of Health Stroke Scale
Methods
Study Design
This was a phase I/II multi-center, open-label study that aimed to evaluate the safety and preliminary ef?cacy of a single intra-venous infusion of marrow-derived allogeneic ischemia-tolerant MSC. Entry criteria appear in Table 1 and in sum describe enroll-ment of adults with radiologically veri?ed chronic stable ischemic stroke and substantial impairment and functional de?cits. Patients were followed for one year after MSC infusion. The study made no restrictions on, and did not provide any forms of, medication or therapy (occupational, physical, or speech) during the follow-up year after infusion. All patients signed consent in accordance with local Institutional Review Board approval. This study was approved by the Food and DrugAdministration and was registered at clinical-trials.gov. The data that support the ?ndings of this study are avail-able from the corresponding author on reasonable request.
The study occurred in 2 parts, with part 1 being a dose-escala-tion study and part 2 being an expanded safety study based on part 1 ?ndings. Part 1 consisted of 3 cohorts (n=5 per cohort) enrolled sequentially in a dose-escalation manner, with subjects receiving one of 3 doses based on body weight, with a maximum dosage of 150 million cells. Cohort 1 received 0.5 million cells/kg of body weight; Cohort 2, 1.0 million cells/kg; and Cohort 3, 1.5 million cells/kg. The dose-escalation plan in part 1 required a review by the Data Safety Monitoring Board once the 5 subjects in Cohort 1 were treated and evaluated through study day 10. If safety was established, Cohort 2 was to proceed at the next highest dose, followed by a similar safety review before escalation to the highest dose in Cohort 3. Part 2 aimed to enroll an additional minimum of 20 subjects at the highest safe dose level determined in part 1. An additional interim review was conducted by the Data Safety Monitoring Board after the ?rst 5 patients were treated in part 2. Detailed stopping rules appear in the online-only Data Supplement (see Stopping Rules and Determination of Maximum Tolerated Dose).
The target dose of 1.5 million cells/kg corresponds to allometric scaling from animal studies. Our meta-analysis of preclinical studies of MSC after experimental ischemic stroke10 identi?ed 9 rodent stud-ies that transfused MSC using the intravenous route in the post-acute period. In each study, MSC provided substantial behavioral gains (effect sizes >1.0), using doses ranging from 3.6 to 12.4×106 MSC/kg body weight (mean dose of 10.1×106 MSC/kg). The approach to al-lometric scaling from animals to humans recommended by the Food and Drug Administration 18 uses a body surface area normalization, which for the mean value in rodents yields a comparable human dose of 1.6×106 MSC/kg.
Cell Manufacturing and Shipping
Manufacturing of MSC was performed at the GMP-compliant fa-cility of the sponsor, Stemedica Cell Technologies, Inc (San Diego, CA). MSC were grown from the bone marrow of a single human donor and are from the same batch used in prior preclinical19,20 and clinical21 studies. Cells were grown under low oxygen (5%) condi-tions. Such ischemia-tolerant MSC have advantages compared with those grown under normoxic conditions, for example, showing higher proliferation rate, expression of stem cell-related genes, production of key cytokines, and migration activity.21,22 Cells were harvested at passage 4 and expressed CD105, CD73, and CD90 surface markers, consistent with the International Society for Cellular Therapy de?-nition.23 Cells were cryopreserved by suspending in Cryostar CS10 freezing medium (BioLife Solutions, Bothell, WA) then stored in the vapor phase of liquid nitrogen. This parent cell bank was then tested for quality control including cell count, viability, appearance, and quantitative polymerase chain reaction for viruses including HIV, Epstein-Barr virus, cytomegalovirus, hepatitis B virus, parvovirus B19, and hepatitis C virus. Cryovials were shipped at =-150° C in a vapor phase liquid nitrogen shipper with temperature monitor.
Infusion of Investigational Product
Each site’s pharmacy prepared MSC for infusion per a study-pro-vided protocol. Cryovials (the number of which was based on the dose to be infused) were thawed and MSC were washed in, and then suspended in, Lactated Ringer’s solution at a concentration of 1×106 cells/mL using one to three 60 mL syringes. The suspension then un-derwent ?nal testing before being released for intravenous infusion, consisting of cell count, endotoxin, Gram stain, and review of appear-ance. Cell count was performed using 0.1% Trypan Blue and a hema-cytometer, which also yielded % cell viability. The minimum percent cell viability was required to be =70% for the cells to be released. A sample was also sent for subsequent sterility testing. After release by the pharmacy, the ?nal formulation was stored at 2° to 8°C and infused within 8 hours of preparation.
MSC Administration
Before MSC infusion, a 0.1 mL aliquot of the ?nal MSC formulation was injected intradermally; any subject showing a positive reaction (eg, wheal with erythema) would not be infused. Cells were admin-istered intravenously via metered-dose syringe pump at 2 mL/min. Patients remained in the inpatient telemetry unit for observation until clinically stable.
Patient Assessments
Patients had frequent monitoring until discharged from the telem-etry unit. After discharge, patients had safety evaluations on day 2, 3, 4, and 10, then again on month, 1, 3, 6, 9, and 12. Adverse events were coded according to the MedDRA adverse event dic-tionary. The relationship that adverse events had to the investiga-tional product was assessed by the site investigator. Patients were followed for one year using tests of behavior, serology, blood chem-istry and cell counts, electrocardiogram, urine, and CT of chest, abdomen, and pelvis. The full schedule of assessments appears in Table SI in the online-only Data Supplement.
Statistics
The primary study end point was safety and tolerability, evaluated in all subjects who received any portion of an infusion, and determined by the incidence/severity of adverse events, clinically signi?cant changes on laboratory and imaging tests, vital signs, and physical plus neurological examinations. Four secondary end points were scored serially to derive preliminary estimates of ef?cacy: National Institutes of Health Stroke Scale, Barthel Index (BI), Mini-Mental Status Exam, and Geriatric Depression Scale. For each, the change from baseline was evaluated using Wilcoxon signed-rank test, with primary analysis of preliminary ef?cacy being change from baseline to 6 months post-infusion, and analysis including all subjects who re-ceived an infusion except for one subject who failed to return after the day 10 visit for all visits (except for month 9 follow-up). For any sub-ject missing 6-month data, 9-month or 12-month data were substituted for this analysis, otherwise missing data were not imputed. Data were analyzed using R statistical software. Given the exploratory nature of this study, sample size was selected as appropriate for detection of any safety concerns in an early phase clinical trial.
Results
Subjects
Of 50 subjects who seemed eligible on prescreening, 36 were enrolled and received treatment from March 14, 2011 to December 15, 2016 (Figure and Table 2). There were 13 subjects enrolled at the University of California, San Diego, 19 subjects at Arizona, and 4 subjects at the University of California, Irvine. Interim safety reviews disclosed no con-cerns, and so 5 subjects received 0.5×106 cells/kg in part 1/ Cohort 1, 5 subjects received 1.0×106 cells/kg in part 1/Cohort 2, 5 subjects received 1.5×106 cells/kg in part 1/Cohort 3, and all 21 subjects in part 2 received 1.5×106 cells/kg. For the 15 subjects in part 1, 12 completed the study, 2 died of unrelated causes (coronary artery disease 6 months post-infusion and sepsis 1 month after infusion), and 1 was lost to follow-up after day 10 (reappearing only for the month 9 follow-up visit). For the 21 subjects in part 2, 19 completed the study, 1 was lost to follow-up after month 6, and 1 was withdrawn by the site PI after month 6 due to treatment with another in-vestigational product. Of the 36 subjects enrolled, the planned dose was delivered within 2 mL (ie, within 2×106 cells) of the target in 26 subjects, whereas in 10 subjects a median of 7.6 (interquartile range, 4.4–10.25) mL (ie, 7.6×106 cells) was not infused as planned, which represented a median of 6.5% (5.3– 9.8) of the intended dose. A total of 179 protocol deviations were reported, mainly related to scheduling study visits or study testing (Table SII in the online-only Data Supplement).
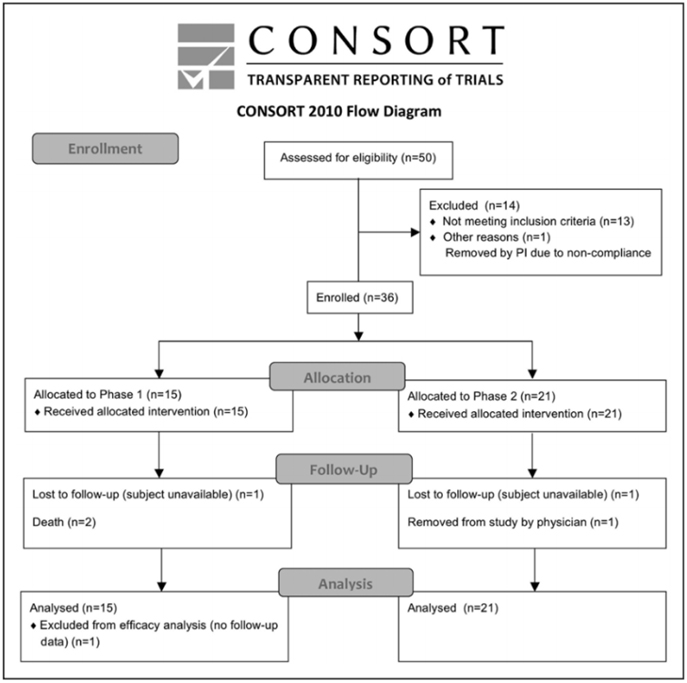
Figure. CONSORT diagram.
Table 2. Baseline Subject Characteristics
| Part1 | Part 2 | Total | |||
| Cohort 1 | Cohort 2 | Cohort 3 | |||
| n | 5 | 5 | 5 | 21 | 36 |
| Sex | |||||
| Male | 5 (100%) | 4 (80%) | 4 (80%) | 14 (66.67%) | 27 (75%) |
| Female | 0 (0%) | 1 (20%) | 1 (20%) | 7 (33.33%) | 9 (25%) |
| Age, y | 50.8:±9.8 [40—62] | 56.8±11.1 [39—69] | 68.8±11.58 [53—84] | 62.8±9.2 [51—83] | 61.1±10.8 [39—84] |
| Race | |||||
| White | 4 (80%) | 3 (60%) | 5 (100%) | 17 (80.95%) | 29 (80.56%) |
| Asian | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (2.78%) |
| American Indian/Alaskan Native | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (2.78%) |
| Native Hawaiian/Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Black | 1 (20%) | 0 (0%) | 0 (0%) | 1 (4.76%) | 2 (5.56%) |
| Other | 0 (0%) | 0 (0%) | 0 (0%) | 3 (14.29%) | 3 (8.33%) |
| Ethnicity | |||||
| Hispanic or Latino | 0 (0%) | 0 (0%) | 0 (0%) | 2 (9.52%) | 2 (5.56%) |
| Non-Hispanic or Non-Latino | 5 (100%) | 5 (100%) | 5 (100%) | 19 (90.48%) | 34 (94.44%) |
| Living situation | |||||
| At home | 5 (100%) | 5 (100%) | 3 (60%) | 19 (90.48%) | 32 (88.89%) |
| In a living facility | 0 (0%) | 0 (0%) | 2 (40%) | 2 (9.52%) | 4 (11.11%) |
| Time from stroke to infusion, y | 1.6±0.9 [0.6—2.9] | 7.7±5.0 [1.1—14.5] | 4.1±2.2 [1.7—7.0] | 4.0±5.0 [0.7—24.8] | 4.2±4.6 [0.6—24.8] |
Values are counts (%) else mean±SD. Values in brackets indicate range.
Safety
A total of 15 serious adverse events were reported.These were wide-ranging in nature, for example, infections, vascular dis-orders, and pain syndromes (for full details, see Table SIII in the online-only Data Supplement). All serious adverse events were deemed unrelated or unlikely related to the investiga-tional product. A total of 109 adverse events were reported, of which 2, both mild, were considered by the site investigator to be possibly related to the investigational product: one uri-nary tract infection and one report of intravenous site irrita-tion. Both adverse events recovered completely.
Study testing disclosed no safety concerns. No subject showed a preinfusion positive reaction to intradermal testing. Serial physical exams and blood testing did not disclose any signi?cant ?ndings. Only one of the serial electrocardiograms was thought to have clinically signi?cant ?ndings, in a subject with moderate intraventricular conduction delay, only at the 1-month follow-up visit. Similarly, across serial CT scans of the chest, abdomen, and pelvis, only one was considered clinically signi?cant, a soft tissue density in the anterior abdominal wall seen at 6-months that was stable when reimaged at 12-months.
Behavioral Effects
Across all subjects, improvements were seen in National Institutes of Health Stroke Scale, BI, Mini-Mental Status Exam, and Geriatric Depression Scale scores at both the 6-month and the 12-month follow-up visits (Table 3). These were statistically signi?cant, generally stable over time, and clinically modest in magnitude. Most ?ndings would sur-vive correction for multiple comparisons. Changes in the BI suggest clinical utility, with a 6.8 point gain by 6-months that grew to a 10.8 point gain by 12-months post-infusion (P<0.001), and with the proportion of patients achieving ex-cellent functional outcome (Barthel score =95) increasing from 11.4% (4/35) at baseline to 9/33 (27.3%) at 6-months to 35.5% (11/31) at 12-months.
Discussion
Stroke is a major cause of human disability. This can be reduced by acute therapies that are introduced in the early hours poststroke to reduce initial injury, and by restorative therapies that are introduced days, months, or years poststroke to promote neural repair. Allogeneic MSC show substantial favorable effects in preclinical studies, including when intro-duced via the intravenous route.10 The current study found a single intravenous infusion of allogeneic MSC to be safe and potentially associated with functional improvement.
The current study is the largest trial of intravenous MSC in patients with chronic stroke and the ?rst to evaluate alloge-neic MSC therapy in this population. It is also the ?rst human stroke study to evaluate MSC grown under hypoxic conditions, which favorably affects cell proliferation, gene expression, cy-tokine production, and migration.21,22 Intravenous infusion of MSCwasfoundtobesafein36patientswhohadchronicstroke with substantial functional de?cits. Across 3 escalating doses, treatment-related adverse events were infrequent, mild, and transient. Serial assessments of exam, laboratory testing, elec-tro cardiogram, and CT scans of chest/abdomen/pelvis disclosed no safety concerns, with limited subject dropout. These results are consistent with the overall excellent safety record that MSC have in clinical trials of human subjects across numerous non-cerebrovascular diagnoses15,24–27 and in stroke trials.11–13,16,17,28,29
Patients with stroke in the chronic stage generally show functional decline; however, enrollees in the current study showed 12 months of continued functional improvement. In general, recovery from stroke-related de?cits shows a bimodal time course. Initially, most stroke survivors show some degree of spontaneous recovery, for example, during the initial months for the motor system.30 Within a year of stroke onset, however, a signi?cant decline in function is commonly seen.31–34 This is signi?cant given that few treat-ment options are available to improve function in patients in the chronic phase of stroke. In the current study, behav-ioral gains were seen, though were modest in magnitude. However, a 2-point improvement in the National Institutes of Health Stroke Scale score (Table 3) in the setting of chronic stroke, if veri?ed in a larger controlled study, might be regarded as important. Also, the mean gain in BI from base-line grew to 10.8 points by 12 month-poststroke (P<0.001), higher than the BI minimal clinically important difference of 9.25 points.35 Furthermore, the proportion of patients with an excellent functional outcome (BI score =95) increased from 11.4% at baseline to 27.3% at 6-months and to 35.5% at 12-months (Table 3). This 12-month period of continued functional improvement is consistent with preclinical studies examining the distribution of systemically administered MSC: intravenous MSC given early after stroke initially localize to lungs then spleen, then increase within the region of brain ischemia,36 and by 30 days poststroke are concen-trated in the peri-infarct region.37 At one year, most surviving MSC are in the peri-infarct region, with very few present in other organs.38 Patients also showed signi?cant improvement in the Mini-Mental Status Exam and Geriatric Depression Scale (Table 3), changes that were largely sustained at 12 months post-infusion, suggesting that MSC have broad effects on brain function. These ?ndings require veri?cation in a larger, controlled study but raise hope that this interven-tion could improve functional status in the chronic stroke setting. Future studies might also incorporate modality-spe-ci?c outcome measures30 to provide more granular assessments of behavioral gains in individual neural systems.
Table 3. Behavioral Change Over Time
| Mini-Mental Status Exam score | n | P Value | |
| Baseline | 24.2±6.0 | 35 | |
| Change to 6 mo | 1.8±2.8 | 32 | <0.001 |
| Change to 12 mo | 1.3±2.7 | 31 | 0.017 |
| NIHSS score | |||
| Baseline | 8 [6.5 to 10] | 35 | |
| Change to 6 mo | -1 [-2.25 to 0] | 32 | <0.001 |
| Change to 12 mo | -2 [-3.5 to -0.5] | 21 | <0.001 |
| Geriatric depression scale score | |||
| Baseline | 5.1±3.5 | 35 | |
| Change to 6 mo | -1.6±3.8 | 32 | 0.015 |
| Change to 12 mo | -1.4±3.8 | 31 | |
| Barthel Index (score) | |||
| Baseline | 65±28.7 | 35 | |
| Change to 6 mo | 6.8±11.4 | 33 | 0.002 |
| Change to 12 mo | 10.8±15.5 | 31 | <0.001 |
| Barthel Index (% =95) | |||
| Proportion at baseline | 4 (11.4%) | 35 | |
| Proportion at 6 mo | 9 (27.3%) | 33 | 0.015 |
| Proportion at 12 mo | 11 (35.5%) | 31 | 0.01 |
Values are mean±SD or median (interquartile range) across all enrollees. Specific data for part 1 and part 2 appear in Table SIV in the online-only Data Supplement. NIHSS indicates National Institutes of Health Stroke Scale.
Meta-analysis of MSC effects in animals with experimental ischemic stroke10 showed large effect sizes that remained sub-stantial after adjusting for potential publication bias and was robust across species, delivery route, time of administration in relation to stroke, and dose. The longest time period when MSC have been introduced poststroke in preclinical studies is 1 month39 or 4 to 6 weeks40 post-infarct. The current ?ndings in patients who were many months poststroke (Table 2) sug-gest the need for bidirectional translation, that is, translation of bedside experience to inform preclinical studies.41,42
There are several strengths to this study. Enrollees had substantial functional de?cits in the chronic stage of stroke, a population that numbers in the millions, for whom treatment options remain limited. The infused cells were allogeneic, an approach made possible by the relatively immunoprivileged nature of MSC,14 which eliminates the need for immuno-suppression15 and which, as compared with autologous cell therapies, enables treatment protocols that can be broadly implemented in the stroke population. A dose-escalation study design was used to evaluate safety. Cell culture was limited to 4 passages, a potential advantage given that higher number of passages (and thus cell divisions) adversely affect MSC features such as proliferation, differentiation, homing, and viability.43–45 Safety was assessed across multiple modalities, including chest/abdomen/pelvis CT and extensive laboratory testing, for a 1-year period.
There are also important weaknesses. As this study was focused on safety, no control group was included, which complicates interpretation of observed behavioral gains (Table 3). Mechanism of action was not studied. Cell thera-pies improving outcomes in the chronic phase likely act via multiple mechanisms that include release of growth factors and anti-in?ammatory effects, and possibly exosomes,46,47 which can be evaluated in subsequent trials. Restorative ther-apies after stroke often provide maximal bene?t when paired with appropriate training,48 but this was not provided in the current safety study.
The current study demonstrated safety of intravenous MSC in patients with chronic stroke who had substantial functional de?cits. Results also suggest functional bene?t, although this requires veri?cation in a controlled study. Together, these ?ndings support further study of intravenous allogeneic MSC in patients with chronic stroke, including evaluation of mech-anism of action.
Sources of Funding
This study was supported by Stemedica Cell Technologies, Inc.
Disclosures
Dr Levy is on the Scienti?c Advisory Board for KOH Robotics. Dr Dib has served as a consultant for J&J Consulting and ISCTR Consulting. Dr Verkh is Chief Regulatory and Clinical Development Of?cer at Stemedica Cell Technologies, Inc. Dr Tankovich is the President and Chief Medical Of?cer for Stemedica Cell Technologies, Inc. Dr Cramer has served as a consultant for Abbvie, Constant Therapeutics, MicroTransponder, Neurolutions, Regenera, SanBio, Stemedica, Biogen, Fuji?lm Toyama Chemical, and TRCare. The other authors report no con?icts.
References
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53.
- Johnston SC, Hauser SL. Neurological disease on the global agenda. Ann Neurol. 2008;64:A11–A12.
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215.
- Lin DJ, Finklestein SP, Cramer SC. New directions in treat-ments targeting stroke recovery. Stroke. 2018;49:3107–3114. doi: 10.1161/STROKEAHA.118.021359
- Cramer SC. Repairing the human brain after stroke. II. Restorative thera-pies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al; EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095
- McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:981–990. doi: 10.1016/j.apmr.2014.10.022
- Dodakian L, McKenzie AL, Le V, See J, Pearson-Fuhrhop K, Burke Quinlan E, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31:923–933. doi: 10.1177/1545968317733818
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilita-tion in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90:498–506. doi: 10.1136/jnnp-2018-319954
- Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501
- Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mes-enchymal stem cells in stroke. Brain. 2011;134(pt 6):1790–1807. doi: 10.1093/brain/awr063
- Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, et al. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferen-tiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896.
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic re-view and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559
- Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and ef?cacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, pla-cebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modi?ed bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. doi: 10.1161/STROKEAHA.116.012995
- Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory Information/Guidances/ucm078932.pdf.Accessed June 22, 2019.
- Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, et al. Intravenously delivered mesenchymal stem cells: sys-temic anti-in?ammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res. 2017;120:1598–1613. doi: 10.1161/CIRCRESAHA.117.310599
- Harach T, Jammes F, Muller C, Duthilleul N, Cheatham V, Zufferey V, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer ’s disease. Neurobiol Aging. 2017;51:83–96. doi: 10.1016/j.neurobiolaging.2016.11.009
- Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic car-diomyopathy: safety and ef?cacy results of a phase II-a randomized trial. Circ Res. 2017;120:332–340. doi: 10.1161/CIRCRESAHA. 116.309717
- Vertelov G, Kharazi L, Muralidhar MG, Sanati G, Tankovich T, Kharazi A. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res Ther. 2013;4:5. doi: 10.1186/scrt153
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for de?ning multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position state-ment. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905
- Devine SM. Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl. 2002;38:73–79.
- Hil?ker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–497. doi: 10.1007/s00423-011-0762-2
- Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophys-iology, translational ?ndings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA. 111.243147
- Figueroa FE, Carrión F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269–277. doi: 10.4067/S0716-97602012000300008
- Tsang KS, Ng CPS, Zhu XL, Wong GKC, Lu G, Ahuja AT, et al. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9:133–143. doi: 10.4252/wjsc.v9.i8.133
- Savitz SI,Yavagal D, Rappard G, Likosky W, Rutledge N, Graffagnino C, et al.A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-Stroke). Circulation. 2019;139:192–205. doi: 10.1161/CIRCULATIONAHA.117.030659
- Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-speci?c outcome measures in clinical trials of stroke recovery-pro-moting agents. Stroke. 2007;38:1393–1395. doi: 10.1161/01.STR. 0000260087.67462.80
- van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37:167–171.
- Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Long-term functional recovery after ?rst ischemic stroke: the Northern Manhattan Study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576
- Wondergem R, Pisters MF, Wouters EJ, Olthof N, de Bie RA, Visser-Meily JM, et al. The course of activities in daily living: who is at risk for decline after ?rst ever stroke? Cerebrovasc Dis. 2017;43:1–8. doi: 10.1159/000451034
- Sennfalt S, Norrving B, Petersson J, Ullberg T. Long-term sur-vival and function after stroke. Stroke. 2019;50:53–61. doi: 10.1161/STROKEAHA.118.022913
- Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair. 2007;21:233– 238. doi: 10.1177/1545968306294729
- Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, et al. Intravenous administration of 99mTc-HMPAO-labeled human mes-enchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 2009;18:1369–1379. doi: 10.3727/096368909X474230
- Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, et al. One-year follow-up after bone marrow stromal cell treatment in mid-dle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic bene?t of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311
- Yasuhara T, Matsukawa N, Hara K, Maki M, Ali MM, Yu SJ, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral de?cits in chronic stroke an-imals. Stem Cells Dev. 2009;18:1501–1514. doi: 10.1089/scd.2009.0011
- Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, et al. Translational stroke research: vision and opportunities. Stroke. 2017;48:2632–2637. doi: 10.1161/STROKEAHA.117.017112
- Armstead WM, Vavilala MS. Translational approach towards deter-mining the role of cerebral autoregulation in outcome after traumatic brain injury. Exp Neurol. 2019;317:291–297. doi: 10.1016/j.expneurol. 2019.03.015
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675– 682. doi: 10.1634/stemcells.22-5-675
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472. CAN-07-5272
- Galipeau J. The mesenchymal stromal cells dilemma–does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002
- Wechsler LR, Bates D, Stroemer P, Andrews-Zwilling YS, Aizman I. Cell therapy for chronic stroke. Stroke. 2018;49:1066–1074. doi: 10.1161/STROKEAHA.117.018290
- Chen J, Chopp M. Exosome therapy for stroke. Stroke. 2018;49:1083– 1090. doi: 10.1161/STROKEAHA.117.018292
- Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(pt 6):1591–1609. doi: 10.1093/brain/awr039







