A new discovery by researchers on how to activate lab-grown beta cells to mature into functioning cells that produce and release insulin in response to glucose take a significant step toward a cell therapy treatment for diabetes.
Difficulties in manipulating beta cells derived from human stem cells to mature beyond the precursor stage into fully functioning insulin releasers has been an on-going challenge for researchers..
However, researchers from the Salk Institute for Biological Studies and a team of researchers have achieved this goal with lab-grown beta cells by activating a protein called estrogen-related receptor γ (ERRγ). Their study findings were recently published in the journal Cell Metabolism.
Self-renewing capacity of human pluripotent stem cells (hPSCs)
Ronald Evans, senior author of the study, titled, “ERRγ Is required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive β Cells,”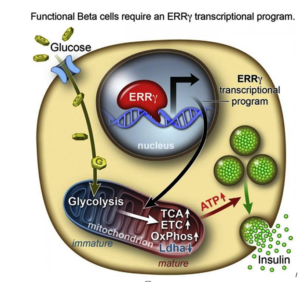 says the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscles cells and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab.
says the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscles cells and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab.
Evans and his research team discovered the answer to the insulin-releasing cell conundrum, and summed it up thusly:
“In a dish, with this one switch, it’s possible to produce a functional human beta cell that’s responding almost as well as the natural thing.”
Evans, a molecular biologist at the Salk Institute, says that to create the different types of cells in the lab, researchers coax the pluripotent stem cells (hPSCs) down the various branching paths that fetal cells normally travel in order to differentiate into the various cell types. However, he explains there are many developmental points in this process, and in the case of lab-grown pancreatic beta cells, research kept getting stuck at an early stage.
Adult beta cells have more ERRγ protein for a very energy-intensive process
In order to determine what might trigger the next step in getting the cells to mature, the researchers compared transcriptomes of adult and fetal beta cells. The transcriptome contains, among other things, the full catalog of molecules that switch genes on and off in the genome, which led them to discover that the nuclear receptor protein ERRγ was more abundant in adult beta cells. The team was already familiar with the protein’s role in muscle cells and had studied its ability to enhance endurance running.
Evans says that in muscles, protein promotes greater growth of mitochondria—the power generators inside cells that accelerate the burning of sugars and fats to make energy.
“It was a little bit of a surprise to see that beta cells produce a high level of this regulator,” Evans says. “But beta cells have to release massive amounts of insulin quickly to control sugar levels. It’s a very energy-intensive process.”
The research team then decided to run some tests to look more closely at what role ERRγ might play in insulin-producing beta cells.
A new era in creating functional, insulin-producing beta cells
 After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
Next they tried to get beta cells made from hPSCs to produce more ERRγ, and it worked! The cells in culture began to respond to glucose and release insulin.
Finally, the team transplanted the lab-grown insulin-producing beta cells into diabetic mice and found that from day one, the cells produced insulin in response to glucose spikes in the animals’ blood.
Evans and the research team were justifiably excited by the results. It appears that just switching on the ERRγ protein is sufficient to get the lab-grown beta cells to mature and produce insulin in response to glucose – both in cultures and in live animals.
Speculating on the implications of their findings, Evans suggests that when a fetus is developing, because it gets a steady supply of glucose from the mother, it does not need to produce insulin to regulate its blood sugar, so the switch is inactive. But, when the baby is born and takes its first breath and takes in oxygen, this activates the switch.
Previous lab attempts to produce beta cells got stuck at the fetal stage. The Salk Institute researchers discovered how to take it to the adult stage, using the same protein that is switched on in nature.
“I believe this work transitions us to a new era in creating functional beta cells at will,” Evans says.
He and his research team now plan to examine how the switch might work in more complex models of diabetes treatments.
The Salk Institute study proceeds another study Medical News Today in which researchers generated mini-stomachs that produce insulin when transplanted into mice.
###







