The objective is to ensure optimal protection against contamination. Cell culture rooms, cryopreservation rooms and even tissue extraction rooms can be designed within the bioclean room with an optimal and biosafe construction that takes into account patient comfort, cell processing as well as the transit of laboratory personnel, doctors and nurses.
WHY BIOCLEAN ROOM?
Aseptic Environment, No contamination.
Air conditioned system.
Optimal Environmental monitoring for cell culture.
MONITORING SYSTEM
Improve and optimize cell culture processes with a monitoring system
for clean room and cell culture equipment We provide equipment to
support processes through a range of different stages in the process.
Emergency Power System
Antistatic Control System
Differential Pressure
Differential Cleanliness
Interlock Systems
MONITORING SYSTEM
Improve and optimize cell culture processes with a monitoring system
for clean room and cell culture equipment We provide equipment to
support processes through a range of different stages in the process.
Emergency Power System
Antistatic Control System
Differential Pressure
Differential Cleanliness
Interlock Systems
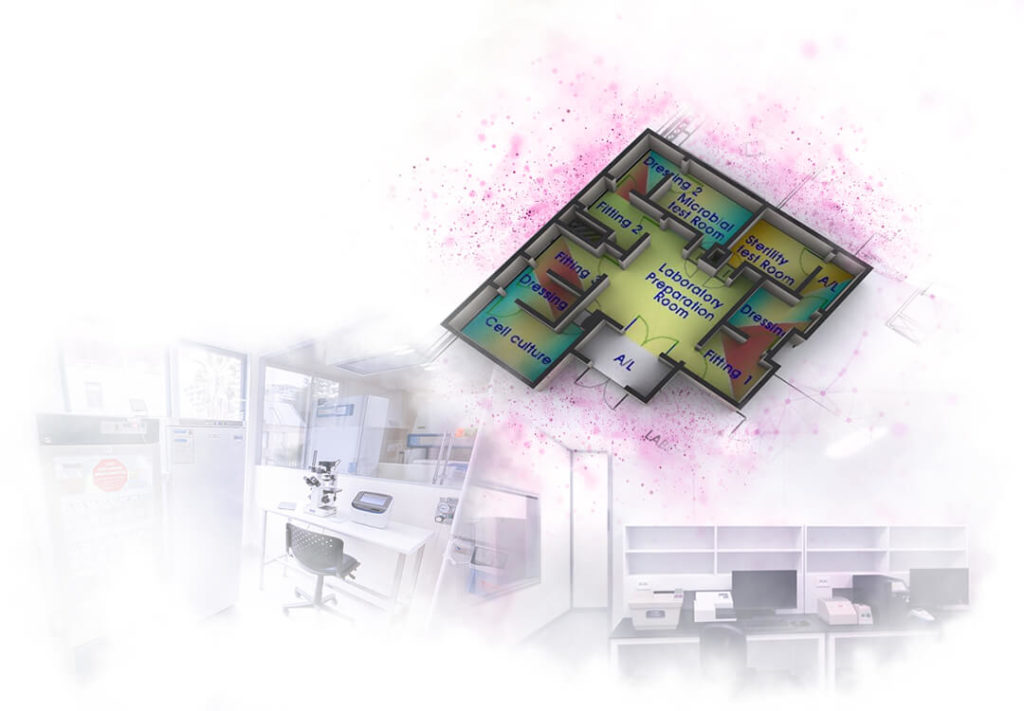
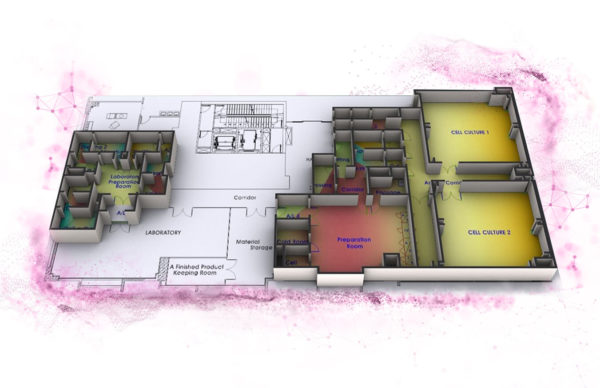
STEM CELL PROCESSING CENTER LAYOUT
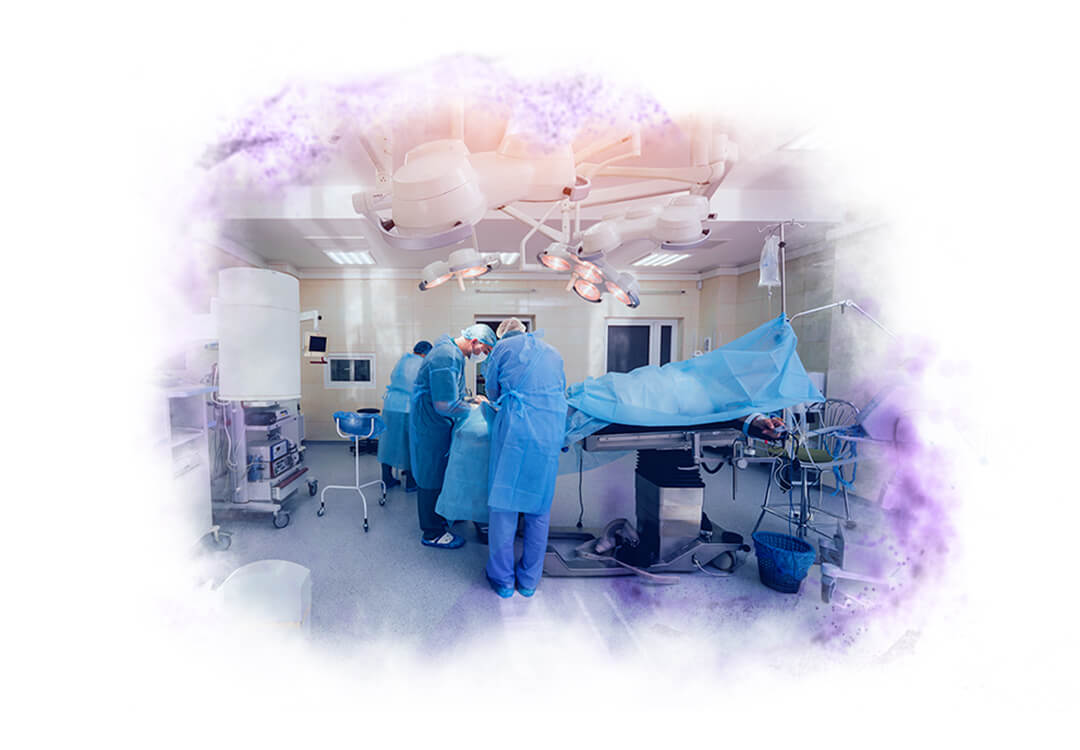
1. PROCEDURE ROOM FOR TISSUE EXTRACTION
The objective of this room is to collect tissue samples from patients in a sterile environment and following safety standards.
1. PROCEDURE ROOM FOR TISSUE EXTRACTION
The objective of this room is to collect tissue samples from patients in a sterile environment and following safety standards.
2. DRESSING ROOM
2. DRESSING ROOM

3. CELL CULTURE & EXPANSION ROOMS
To ensure all cell culture procedures are performed to a standard that will prevent contamination from bacteria, fungi and mycoplasma and cross contamination with other cells lines.
Stem Cell Isolation
Stem Cell Subculture
Stem Cell Culture
3. CELL CULTURE & EXPANSION ROOMS
To ensure all cell culture procedures are performed to a standard that will prevent contamination from bacteria, fungi and mycoplasma and cross contamination with other cells lines.
Stem Cell Isolation
Stem Cell Subculture
Stem Cell Culture

4. CRYOSTORAGE ROOM
Cell Storage
Reagent Storage
Reagent Preparation
LN2 Supply
4. CRYOSTORAGE ROOM
Cell Storage
Reagent Storage
Reagent Preparation
LN2 Supply

5. QUALITY CONTROL AREA
Good laboratory practice requires testing normal and abnormal controls for each process at least daily to monitor the analytical process. If the test is stable for less than 24 hours or some change has occurred which could potentially affect the best stability, controls should be assayed more frequently.
Endotoxin Test
Mycoplasma Test
Aseptic Test
Stem Cells Quality Test
5. QUALITY CONTROL AREA
Good laboratory practice requires testing normal and abnormal controls for each process at least daily to monitor the analytical process. If the test is stable for less than 24 hours or some change has occurred which could potentially affect the best stability, controls should be assayed more frequently.
Endotoxin Test
Mycoplasma Test
Aseptic Test
Stem Cells Quality Test